Managing inventory with expiration dates is a critical challenge for cosmetic brands. Products with limited shelf lives, like mascara or sunscreen, require strict oversight to ensure safety, compliance, and quality. This article explores how modern ERP systems help cosmetic manufacturers tackle these challenges, reduce waste, and maintain compliance with FDA regulations.
Key Takeaways:
- What is Expiry-Controlled Inventory? Managing products by expiration dates to ensure safety and reduce waste.
- FDA Requirements: Cosmetics don’t require labeled expiration dates, but brands must ensure safety through stability testing and compliance.
- Financial Risks: Expired products contribute to $4.8 billion in losses annually in the beauty industry.
- ERP Tools for Cosmetics: Batch tracking, automated alerts, stock age monitoring, and compliance management streamline inventory processes.
- Procuzy ERP Example: Real-time tracking, production planning integration, and automated compliance reduce waste and improve efficiency.
These insights highlight how ERP systems like Procuzy are tailored to the complexities of cosmetic manufacturing, offering tools to manage perishable inventory, meet regulatory standards, and cut losses.
Core ERP Features for Managing Expiry-Controlled Inventory
Modern ERP systems offer cosmetic manufacturers advanced tools to manage inventory with strict expiration requirements. These tools go far beyond basic inventory tracking, addressing the unique challenges faced by the cosmetics industry. Understanding these features can help manufacturers select the right solution and use it effectively.
Batch and Lot Tracking
In the cosmetics industry, precise batch tracking is essential for both safety and regulatory compliance. ERP systems enable real-time tracking of materials, from their arrival at the facility to production, packaging, and distribution. This level of detail is critical for managing expiration dates, especially for formulations with multiple ingredients, each with its own quality standards and shelf life.
Batch numbers are assigned to materials, making it easy to follow their journey through production and distribution. Integrated quality control checkpoints ensure that only batches meeting strict standards proceed, with results automatically linked to specific batch numbers. Additionally, ERP systems support dynamic formulations, allowing for adjustments with version tracking to ensure batch records accurately reflect the specifications used during production.
Regulatory compliance is another key aspect of batch tracking. Agencies like the FDA and EU Cosmetic Regulation require detailed documentation of ingredient sources, manufacturing dates, and batch records. This documentation not only ensures safety but also protects the brand by maintaining high standards in an industry reliant on complex formulations and raw material variability.
Automated Alerts and Stock Age Monitoring
Building on batch tracking, ERP systems use automated alerts to help manufacturers manage inventory proactively. These alerts notify teams about low stock levels, reserved items, or back-ordered products while also tracking expiration dates to prevent products from lingering too long on shelves.
The system provides real-time visibility into inventory levels, helping identify slow-moving items and reducing overstocking. Forecasting tools optimize production schedules and quantities, ensuring inventory aligns with demand.
Stock age monitoring is especially valuable for products with varying shelf lives. ERP systems can prioritize orders based on expiration dates, customer importance, or order size, ensuring older inventory is used first. This approach aligns with the "first expired, first out" model, which helps minimize waste.
With ERP systems, inventory accuracy can improve significantly – from 60–70% to over 90%. This improvement not only reduces waste but also enhances cash flow management. Additionally, trend analysis features allow manufacturers to study historical data, identifying patterns in product movement. This insight supports better inventory strategies, accounting for seasonal trends, product lifecycles, and market demands.
Automated Compliance Management
ERP systems also simplify regulatory compliance through automation, ensuring that cosmetic brands meet the complex requirements of the U.S. market. These systems enforce digital controls over safety documentation, ingredient traceability, facility registration, and adverse event reporting.
Compliance automation handles multiple tasks simultaneously. For example, ERP systems generate real-time batch records, validate ingredients against global regulatory lists, produce multilingual labels, and track deviations using Corrective and Preventive Action (CAPA) workflows. By reducing the risk of human error, these systems provide actionable insights through analytics.
Take Niko Cosmetics as an example. After implementing Mar–Kov ERP, the company cut its regulatory approval cycle time by 35%, eliminated 90% of manual batch documentation errors, and launched three new SKUs into the Canadian and EU markets without compliance delays.
"V5 digitized everything from batch records to adverse event tracking. We’re no longer chasing compliance – we’re ahead of it." – Head of Regulatory, Cosmetics Manufacturer
Automated compliance systems also ensure up-to-date records for product listings and facility registrations. They enforce Good Manufacturing Practices (GMP) by digitizing processes like line clearance, batch step confirmations, equipment calibration logs, and label reconciliation. These features not only protect brands but also build consumer trust – an essential factor, as 67% of consumers say they would stop buying from a brand after a safety issue.
Integration capabilities further enhance compliance features. Product information, including ingredients, expiration dates, and certifications, is automatically updated across all platforms, reducing the risk of outdated data appearing in the system.
ERP Customization for Cosmetic Brands: Procuzy‘s Approach
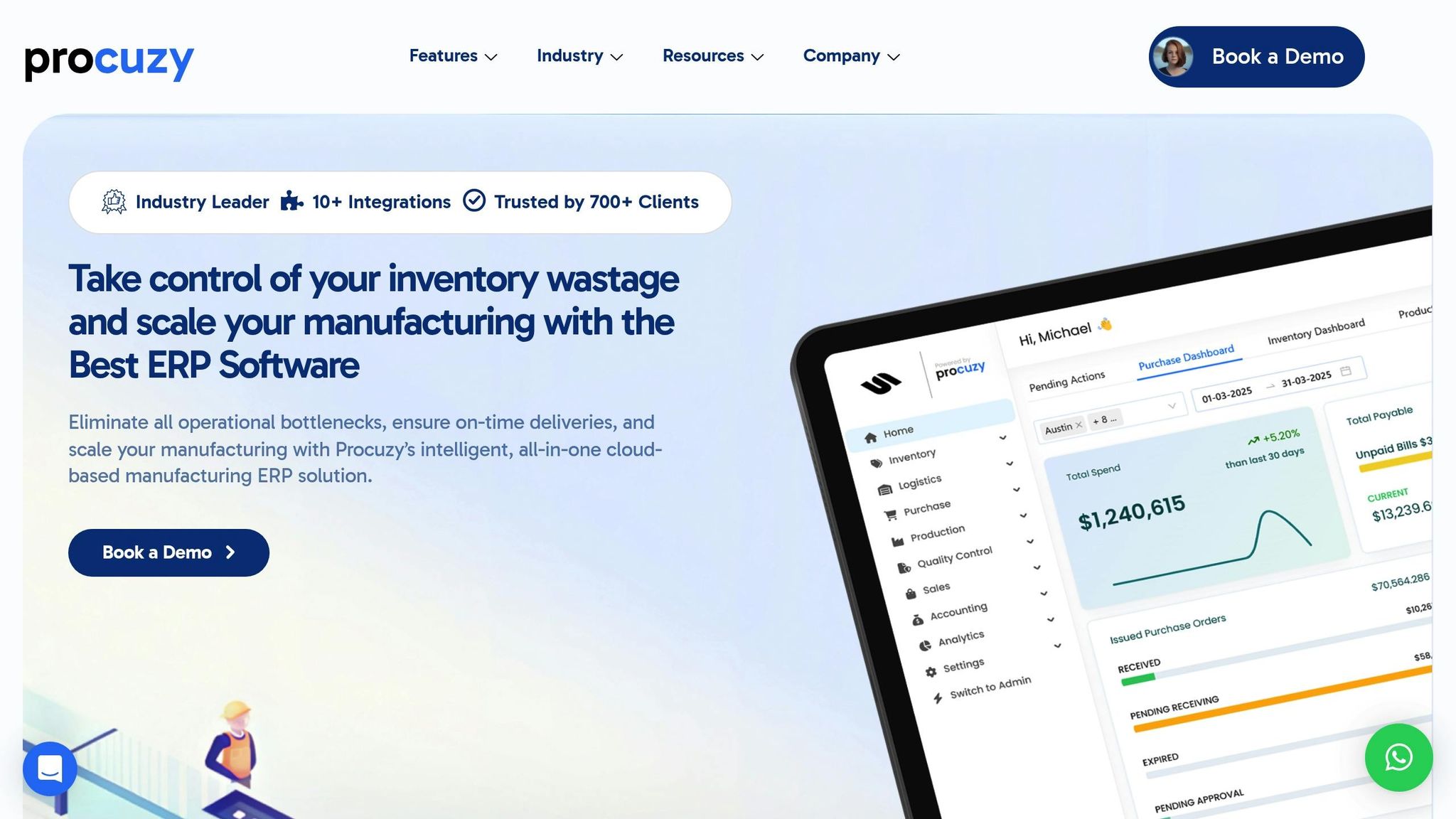
Procuzy takes its well-established ERP foundation and adapts it to meet the specific needs of cosmetics manufacturers. The cosmetics industry faces unique hurdles, from managing complex formulations to adhering to strict regulations. Procuzy’s cloud-based ERP system is designed to tackle these challenges head-on, offering tailored solutions that streamline operations, ensure compliance, and enhance efficiency.
Shelf Life Tracking and Batch Management
Procuzy’s inventory management module is built with the cosmetics industry in mind, providing tools for real-time shelf-life tracking and stock monitoring. By using unique batch identifiers, the system seamlessly combines real-time tracking with advanced shelf-life analytics. This feature is crucial for managing formulations that include multiple ingredients, each with its own expiration date and quality standards.
Dynamic stock thresholds, informed by historical data, automatically adjust inventory levels to meet demand. Automated quality checks quickly identify any deviations, reducing waste and ensuring product integrity. Additionally, the system’s ability to monitor expiration dates and send automated alerts helps prevent losses and guarantees that only fresh products make it to consumers’ hands.
Connecting Expiry Data with Production Planning
Procuzy goes a step further by linking expiry data directly with production planning tools, helping manufacturers cut waste by up to 60%. This integration is especially beneficial for handling seasonal items and limited-edition collections. For instance, when inventory moves slowly and nears its expiration date, the system can adjust production schedules to prioritize the use of older materials. This reduces waste and ensures efficient use of resources.
The platform also provides real-time visibility into inventory levels, expiration dates, and sales forecasts, enabling production planners to determine the best batch sizes and manufacturing timelines. By overseeing the entire Bill of Materials, Procuzy ensures raw ingredients are ordered and used in the right sequence. These features have led to a significant reduction in material waste, saving manufacturers time and money. Additionally, the system includes robust security measures to protect sensitive data, ensuring compliance and operational integrity.
User Access Controls and Data Security
In addition to inventory and production tools, Procuzy prioritizes data security and access management to maintain compliance and operational reliability. Role-based access controls limit employees to the information they need for their specific roles. For example, production staff can view batch tracking and quality control data, while procurement teams access supplier and purchase order details. Sensitive formulation and compliance data are encrypted both in transit and at rest, ensuring maximum protection.
Procuzy’s cloud-based architecture allows real-time access to critical information while maintaining detailed audit trails that log all system interactions. The platform also integrates seamlessly with existing tools like accounting systems and CRM platforms, providing a secure and interconnected ecosystem.
Real-world success stories highlight the system’s impact. For example, Shivanika Foods has fully automated its processes to improve efficiency and visibility, while Staschem has enhanced its production workflow with real-time batch tracking and inventory optimization. These capabilities not only protect sensitive data but also ensure compliance with FDA regulations. Procuzy’s system meticulously tracks ingredient sources, manufacturing dates, and quality test results, while restricting access to authorized personnel, safeguarding critical compliance data every step of the way.
Steps for Successful ERP Implementation in Cosmetics Manufacturing
Rolling out an ERP system in cosmetics manufacturing requires careful planning to keep operations running smoothly and achieve a strong return on investment. Managing expiry-controlled inventory adds an extra layer of complexity, making a structured approach essential. With the right steps, your team can transition seamlessly to the new system while maintaining compliance and operational efficiency.
Data Migration Best Practices
Migrating inventory and batch data to a new ERP system is one of the most critical parts of the process. Errors here can derail the entire project, so it’s crucial to follow proven strategies.
Start by defining the scope of your data migration. Pinpoint all data sources, such as inventory records, quality control logs, batch tracking systems, and compliance documentation.
Bring together a cross-functional team with members from inventory, quality control, production, and IT. This group will ensure that all essential data is accounted for and properly handled during the migration.
Standardize your data. This means creating uniform conventions for batch numbers, expiration dates, and other key details. In an industry where precise tracking of ingredients and expiration dates is vital, this step cannot be overlooked.
Thoroughly test, clean, and reconcile data to ensure accuracy. Running parallel systems during the transition can help you catch and fix discrepancies before going live.
Choose an ERP vendor experienced in handling the specific needs of cosmetics manufacturing, particularly batch tracking and expiry-controlled inventory. Discard outdated or redundant information to save resources, and map your current data structure to the new ERP system to ensure everything aligns properly.
Staff Training and Change Management
Training is a cornerstone of successful ERP implementation. It helps employees understand the system and adapt to new workflows – especially important in managing expiry-controlled inventory, where precision and compliance are non-negotiable.
"No ERP implementation can succeed without pre- and post-launch training that teaches employees how to use the system to its full potential."
Tailor training programs to the needs of each department. For example, inventory managers should focus on batch tracking and expiration monitoring, while production teams need to learn about real-time inventory updates. Quality control staff require specialized training on compliance reporting and audit trails.
Form a cross-functional change management team with representatives from inventory, production, quality assurance, procurement, and senior leadership. This team can identify potential resistance to the new system and develop strategies to address concerns before they escalate.
Hands-on training in a sandbox environment is invaluable. Simulate real-world scenarios like managing seasonal inventory, handling product recalls, or tracking ingredient batches. This helps employees see how the system will impact their daily tasks.
Boost user adoption with in-app guidance and support during regular workflows. For instance, the Renewable Energy Group (REG) saw impressive results with this approach, including a 50% reduction in time-to-proficiency for new users, a 3-month decrease in ERP adoption time for new hires, and a 600% drop in daily ERP-related IT help desk tickets.
Lastly, a clear communication plan is key. Regular updates on implementation progress, training schedules, and system benefits can help keep the team motivated and reduce resistance to change.
Using ERP Data for Continuous Improvement
Once the ERP system is fully operational and employees are comfortable using it, the focus shifts to leveraging its data for ongoing improvements. Use the system to optimize inventory management and cut down on waste. By monitoring inventory levels and analyzing key performance indicators (KPIs), you can fine-tune operations over time.
Pay close attention to metrics tied to expiry-controlled inventory, such as waste percentages by product category, average inventory age, and compliance audit results. These insights can guide adjustments to inventory policies, reorder points, and safety stock levels, helping to improve shelf-life management and reduce waste.
Regular feedback from ERP users is also critical. Monthly feedback sessions can uncover issues and provide opportunities to refine system configurations and training programs.
Monitoring user behavior within the ERP system can highlight areas where additional support or training may be needed. A data-driven approach to continuous improvement ensures that your ERP system keeps delivering value well beyond the initial rollout.
sbb-itb-a748ddd
Compliance and Market Requirements for US Cosmetic Brands
Running a cosmetic brand in the United States means dealing with a maze of regulations while keeping up with consumer expectations. Recent changes in legislation, paired with heightened awareness about product safety and transparency, have made this landscape more demanding. To navigate these challenges effectively, businesses need ERP systems designed to handle compliance and transparency requirements.
Following US FDA Regulations

The FDA takes a hands-off approach when it comes to cosmetics, placing the responsibility for safety squarely on manufacturers. For instance, while the FDA doesn’t mandate expiration dates on cosmetic labels, manufacturers must ensure their products remain safe to use. This puts the onus on brands to determine and document shelf life.
Some products, like sunscreen-infused makeup, fall under dual-class regulations and require stability testing along with expiration dates. The stakes have been raised further with the Modernization of Cosmetics Regulation Act of 2022 (MoCRA), which mandates that cosmetics sold in the U.S. adhere to Good Manufacturing Practices (GMPs).
To meet these evolving FDA requirements, maintaining detailed records and ensuring batch traceability is essential. The FDA actively monitors cosmetic imports and can block products deemed adulterated or misbranded. Additionally, the Fair Packaging and Labeling Act (FPLA) gives the FDA authority over labeling and content disclosures, making it critical for businesses to integrate labeling compliance into their inventory systems.
Setting Up ERP for US Market Standards
ERP systems must be tailored to meet U.S. regulatory and business norms. This includes using the MM/DD/YYYY date format, displaying currency with the dollar sign ($) and proper comma separators, and recording temperatures in Fahrenheit – standard practice in most U.S. facilities and logistics operations.
Temperature monitoring is especially important, as storage conditions directly affect product stability. For example, mascara might need to be discarded within 2–4 months of opening due to contamination risks or breakdown of preservatives.
Procuzy ERP is an example of a system built to meet these needs. It tracks batches with unique IDs, monitors expiration dates, and even sends alerts for perishable or regulated items. It also accounts for factors like exposure to moisture, temperature changes, sunlight, and air, which can impact shelf life.
Brands using systems like Procuzy have reported notable improvements. Toral Patel from Marico shared:
"The Procuzy team is very helpful and responsive, and with improved data accuracy, planning efficiency has significantly increased."
Meeting US Consumer Expectations
American consumers are becoming more discerning, with growing demands for transparency and safety in the products they buy. Surveys show that 80% of consumers want stricter regulations, and 90% prefer clean, safe products. Additionally, 58% are willing to pay a premium for beauty products that ensure ingredient safety and ethical sourcing, while 67% actively check labels for safety certifications before purchasing.
Consumer habits have shifted significantly, with over 70% now reviewing ingredient lists and 74% prioritizing organic ingredients in personal care products. However, trust remains an issue – only 9% of Americans fully trust voluntary product labels.
"Consumers are demanding more transparency, and regulatory bodies are tightening safety and sustainability requirements from the beauty industry." – SEA Vision
To meet these expectations, brands need ERP systems that provide real-time, verifiable data. Such systems should document ingredient sourcing, maintain scientific evidence of product safety, and support third-party testing and certification processes to build consumer trust.
David Trosin, Senior Director of NSF‘s Global Certification team, highlights the importance of credibility:
"Despite this growing demand, greenwashing is more prevalent than ever and consumer trust in voluntary organic labels is lacking, underscoring the importance of third-party testing and certification."
Procuzy users have found success in addressing these transparency challenges. Saurav A. from Daily Objects noted:
"With Procuzy as a CXO, we are able to get meaningful insights on our manufacturing end and reports on work happening at the ground level."
The regulatory landscape is shifting to align with consumer demands. The Modernization of Cosmetics Regulation Act represents a major change for the U.S. cosmetics industry, introducing stricter requirements for registration, labeling, adverse event reporting, and GMPs. To stay competitive, your ERP system must adapt to these changes while delivering the transparency today’s consumers expect.
Conclusion: Managing Inventory with Procuzy ERP
Handling expiry-controlled inventory in the cosmetics industry demands precision and streamlined processes. Procuzy ERP tackles these challenges head-on, helping businesses meet FDA requirements, reduce waste, and turn potential hurdles into opportunities for growth. With its tailored features, Procuzy offers clear advantages for managing inventory effectively.
Key Advantages of ERP for Expiry-Controlled Inventory
Procuzy users have reported impressive outcomes, including a 60% reduction in wastage through precise inventory tracking, 1.5x faster turnaround times thanks to workflow automation, and a 22% decrease in operational costs by optimizing resources.
Real-time inventory tracking eliminates uncertainties about stock levels, while automated alerts prevent both stockouts and overstocking. Batch tracking ensures complete traceability, and automated quality checks maintain consistent standards. For products with varying shelf lives, expiration monitoring becomes essential – not just for safety but also for profitability.
Anurag Satyarth from Eggoz highlighted the impact of Procuzy on their processes:
"I have multiple stages in my manufacturing process and Procuzy’s factory set up module helps me to streamline it and prevent huge wastage at each stage."
Why Cosmetic Brands Choose Procuzy
Procuzy is purpose-built for the unique demands of cosmetic manufacturing. Unlike generic inventory systems, it addresses the complexities of this industry, from managing detailed Bills of Materials to tracking perishable items throughout the supply chain.
Its cloud-based platform integrates data across procurement, inventory, production, and sales, offering a unified view that simplifies coordination across departments and locations. This centralized system is particularly valuable during batch recalls, enabling quick identification of affected retailers and reducing response times. These features directly contribute to better profitability and market adaptability.
Ayush Saxena from Staschem shared his experience with Procuzy:
"Procuzy transformed our production workflow with real-time batch tracking and inventory optimization."
The platform also uses dynamic stock thresholds, adjusting based on historical sales and seasonal trends. This demand forecasting minimizes the risk of running out of popular products while avoiding excess stock of slower-moving items.
Procuzy consistently earns high praise from its users, boasting a 4.7/5 rating on GetApp. Customers frequently highlight its strengths in inventory tracking, analytics, and purchasing workflows. One user even reported a 15% reduction in waste by leveraging insights from Procuzy’s analytics.
Support and customization further set Procuzy apart. As Arpit from Shivanika Foods noted:
"Procuzy automated our entire process flow, centralizing operations tracking while boosting efficiency and transparency."
For cosmetic manufacturers navigating strict regulations and rising consumer expectations, Procuzy delivers more than just inventory management. It provides a robust foundation for achieving compliance, reducing waste, and driving profitability. With its industry-specific tools, proven results, and dedicated support, Procuzy is an excellent choice for brands ready to master expiry-controlled inventory challenges.
FAQs
How can ERP systems like Procuzy help cosmetic brands manage inventory with expiration dates and reduce waste?
ERP systems, like Procuzy, provide cosmetic brands with powerful tools to manage inventory that includes expiration dates. Through features such as real-time tracking, shelf-life monitoring, and batch management, brands can maintain tight control over stock levels and expiration schedules. This helps prevent overstocking and cuts down on product waste.
Procuzy also incorporates lot tracking and the FEFO (First Expiring, First Out) inventory method, ensuring that products closest to expiration are prioritized for sale or use. This approach not only reduces waste but also improves stock rotation and keeps brands in line with regulatory requirements. By automating these critical processes, Procuzy streamlines operations, saving cosmetic manufacturers valuable time and resources.
How does Procuzy ERP help cosmetic brands comply with FDA regulations while managing inventory effectively?
Procuzy ERP is tailored to meet the unique challenges faced by cosmetic manufacturers, particularly when it comes to staying compliant with FDA regulations and managing inventory effectively. The platform offers batch tracking tools to oversee product lifecycles, quality control modules to maintain safety and consistency, and integrates smoothly with regulatory reporting systems for precise and timely submissions.
Additionally, Procuzy enables cosmetic brands to automate alerts for product expiration, streamline shelf-life management, and keep comprehensive records to simplify audits. These features not only make compliance easier but also help cut down on waste and boost overall efficiency in operations.
How can cosmetic brands use ERP systems to improve inventory management and stay compliant with regulations?
Cosmetic brands can benefit significantly from ERP systems, especially when it comes to managing inventory and staying compliant with regulations. By centralizing data on things like product shelf life, batch tracking, and compliance requirements, these systems allow businesses to monitor inventory in real-time. This not only reduces waste but also ensures they meet standards such as FDA guidelines and GMP requirements.
In addition, ERP systems take care of essential tasks automatically. They can generate audit reports, send alerts for products nearing expiration, and handle compliance checks. With these tools, brands can stay ahead by adjusting inventory levels and fine-tuning processes, making their operations more efficient while staying aligned with regulatory demands.
